Iso 13485 internal audit checklist free
ISO 13485:2016 Internal Auditor Training Course

Updating the Quality Plan as the project proceeds The constant monitoring and update of the Quality Plan will be required throughout the project implementation or product manufacture. Full quality system internal audit The timing of the internal audit should be late enough in the quality plan. Five Reasons To Choose Our Templates 1. The elements that form the quality management system are the same. The document is optimized for small and medium-sized organizations — we believe that overly complex and lengthy documents are just overkill for you. Corrective and Preventive actions could be induced by different reasons. Writing clear nonconformity statements Break out Exercise 4 1.
ISO 13485:2016 checklist

The process descriptions or definitions have to contain the specifics and the real how-to facts for each defined process. Why should you use checklists in your internal audit? Training records should include evidence of effectiveness of training, and you should be able to demonstrate competency of the people performing those procedures. For example, rearranging resources, prolong due dates, adding new activities that had been not foreseen previously etc. Are The Templates Suitable For You? The Organizational chart should include the positions with or without concrete names or avatars and the relationships hierarchy between those positions. Classification of the medical device Review the characteristics of your device and determine its classification. These process definitions and rules should be available for relevant employees.
ISO 13485:2016 checklist

Examples of Work Instructions may include directions for using certain computer software or performing a task on an assembly line. You can choose to either send us your information using our contact form to get a free quotation or contact us via phone at 612-208-7845. When you are reviewing your process plans, you can write down what you need to check, and in this way you can make sure that nothing important is forgotten. The Corrective actions should be investigated and the root cause should be determined. If the internal auditor s have been heavily involved in the implementation of the quality system, the Company may decide to hire an external consultant to perform the first internal audit. It will help you to understand each business process in the context of each of the requirements by comparing different activities and processes with what the standard requires.
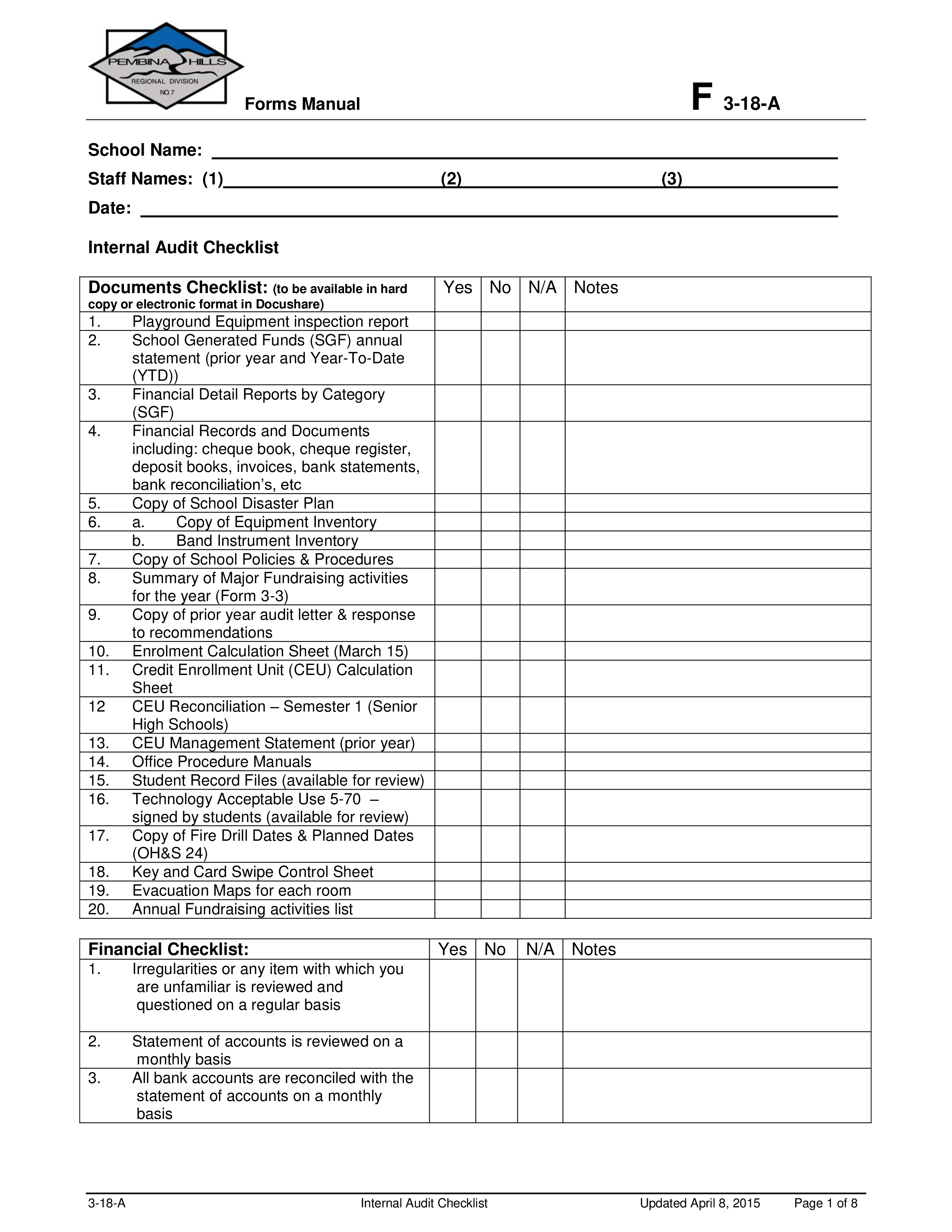
Free ISO Downloads [procedures, checklists, process maps, forms]

An audit checklist is basically a set of questions that the auditor wants to ask, or activities that the auditor wants to witness, in order to verify the planned arrangements as above. All the employees should be aware of the Quality policy and are required to work according to this policy. About 13485Academy 13485Academy is one of the Academies of. All of your personal information, including credit card number, name, and address is encrypted so it cannot be read during transmission. The latter list now becomes the target of your Implementation Checklist. How can you tell if the process is effective? The checklist can include more than just questions; it can also include statements from the procedures that the auditor wants to check.
ISO 13485:2016 Internal Auditor Training Course
Advisera specializes in helping organizations implement top international standards and frameworks such as , , , , , , , , and. All audit findings should be recorded in the Internal audit report. This audit checklist may be used for element compliance audits and for process audits. The quality policy should be promoted throughout the company. The audit checklist stands as a reference point before, during and after the internal audit process.
13 ISO Checklists [ISO ISO 9001, 14001, 45001]

Organizational structure Establish the draft of the company's structure, in a so-called Organizational chart. Preventive actions can be implemented in almost any case after the Corrective action. A management review doesn't have to be a huge meeting with all the managers, external experts, etc. Medical devices - Quality management systems - Requirements for regulatory purposes represent the requirements that the medical device manufacturers must incorporate into their management systems. In order to determine the possible preventive action, a so-called Root Cause analysis should be performed. Product quality objectives are defined in drawings and specifications, contracts, standards, samples, workmanship standards, and applicable legal and regulatory requirements.
ISO 13485:2016 Internal Audit Checklist

The application of our templates is scalable and generic; regardless of the size and type of organization. A logical checklist is well divided into separate sections, presents logical sets of questions and is intuitive to use. Remember that the checklist is a tool for the auditor, and not something to give the auditee to fill out, so whatever format or questions and statements will be useful for the auditor to make sure that all important parts of the process are checked will work. Selection of Auditors and Subject Matter Experts 3. The core processes might include design and development processes, customer-related processes and production related processes. The outsourced processes should be controlled by the Company.
ISO 13485 internal audit How to create a checklist

Free Quote There are two ways to move forward. Our team has prepared these documents in compliance with all the necessary requirements. A gap analysis of the new requirements is strongly recommended in order to identify realistic resource and time implications. Many companies avoid over-burdening their quality manual by allowing lower-level documents, such as procedures and work instructions to contain the operational detail. You can customize this iso 13485 checklist to make your own internal audit checklist. Work instructions might describe how to operate machinery, how to mix chemicals for a certain process, how to process a purchase order, or anything else that you feel is important enough to document and share.
ISO 13485 2016 Documents with Manual, Procedure, Audit Checklist in English

Purchasing Management has to decide whether there are certain processes that should be outsourced, such as manufacturing, installation, servicing, packaging, etc. Determine the Quality Objectives The quality policy sets the route to Quality objectives that are more concrete goals related to certain processes. Our products are of best-in-class quality. A good quality checklist guides the user. The application of our templates is scalable and generic; regardless of the size and type of organization. This concept is for the process owner to have one or several main measures for their process that will let them know that the process is functioning as expected.
Nba 2k14 modded to 2k16 apk
Dreamweaver download free full version windows 7
Office 16 product key list
